Medsafe natural health products are defined as dietary supplements and herbal preparations that fall under the regulatory oversight of the New Zealand Medicines and Medical Devices Safety Authority (Medsafe). Unlike pharmaceutical medicines, these products are generally regulated under the Dietary Supplements Regulations 1985 and the Medicines Act 1981, requiring that they do not make therapeutic claims to treat or cure diseases unless they have undergone a rigorous consent process to be registered as a medicine.
The Role of Medsafe in New Zealand’s Natural Health Sector
In the landscape of New Zealand’s healthcare, the Medicines and Medical Devices Safety Authority, commonly known as Medsafe, plays a pivotal role in maintaining the safety and quality of products available to consumers. For the NZ Integrated Herbal Medicine & Rongoā Māori Hub, understanding Medsafe’s jurisdiction is fundamental to operating legally and ethically.
Medsafe is a business unit of the Ministry of Health. Its primary mandate is to administer the Medicines Act 1981 and the Medicines Regulations 1984. However, the regulation of Medsafe natural health products occupies a unique space. Unlike prescription medicines, which undergo pre-market assessment and approval, many natural health products enter the market as “dietary supplements” under the Dietary Supplements Regulations 1985.
It is a common misconception that natural products are unregulated. While they may not require the same clinical trial data as a new pharmaceutical entity, they are subject to strict post-market monitoring. Medsafe is responsible for investigating complaints, monitoring adverse reactions, and recalling products that pose a safety risk or contain undisclosed prescription ingredients. Their authority ensures that while New Zealanders have access to natural remedies, that access does not come at the cost of public health safety.
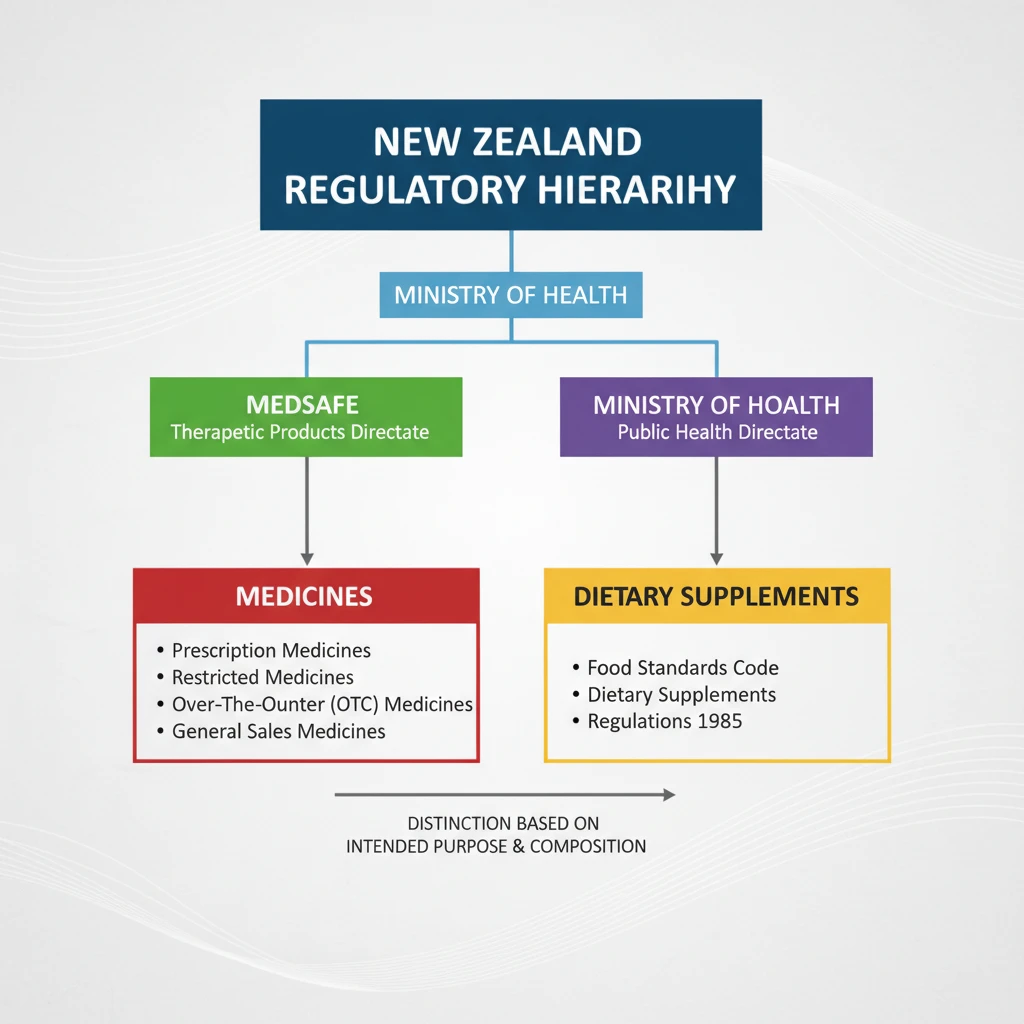
Classifying Natural Health Products: Supplement vs. Medicine
One of the most complex challenges for manufacturers and practitioners of herbal medicine is correctly classifying a product. The distinction determines the regulatory pathway and the level of scrutiny the product will face. In New Zealand, a product containing herbal ingredients is generally classified in one of three ways:
1. Dietary Supplement
Under the Dietary Supplements Regulations 1985, a dietary supplement is defined as any amino acid, edible substance, herb, mineral, synthetic nutrient, or vitamin intended to supplement the diet. Crucially, these products must be intended for oral consumption and cannot be represented as food for ordinary use. If a product fits this definition and makes no therapeutic claims, it is regulated as a supplement, not a medicine.
2. Related Product
A “Related Product” is a category under the Medicines Act. These are products that may have a therapeutic purpose or contain ingredients at doses that exceed those permitted in dietary supplements but are not strictly “medicines” in the conventional pharmaceutical sense. An example might be a high-dose vitamin injection or a throat lozenge with antiseptic properties. These require consent from the Minister of Health to be distributed.
3. Medicine
If a natural health product claims to treat, cure, or prevent a disease, or if it has a pharmacological action that interferes with normal physiological function, it is classified as a medicine. To legally sell such a product, the sponsor must obtain consent for distribution, which involves submitting substantial evidence of safety, quality, and efficacy.
For further reading on the specific definitions within the legislation, you can refer to the official Ministry of Health Medsafe Guidelines.
Compliance Requirements for Manufacturers and Suppliers
For businesses operating within the NZ Integrated Herbal Medicine & Rongoā Māori Hub, compliance is not optional. Failure to adhere to regulations regarding Medsafe natural health products can result in product seizures, fines, and significant reputational damage. The compliance landscape involves several key areas:
Ingredient Permissibility
Manufacturers must ensure that their formulations do not contain scheduled medicines or restricted substances. Medsafe maintains a database of classification decisions. For example, certain herbs that are considered safe in traditional practice may be restricted if they contain alkaloids that are classified as prescription medicines at specific concentrations.
Good Manufacturing Practice (GMP)
While dietary supplements in NZ do not currently mandate a specific Code of GMP under the 1985 regulations, adhering to GMP is the industry standard for ensuring safety. This involves:
- Sanitation: Ensuring the manufacturing facility is hygienic.
- Process Control: Documenting every step of the manufacturing process (Standard Operating Procedures).
- Quality Control: Testing raw materials for identity and purity before use.
- Record Keeping: Maintaining batch records to facilitate recalls if necessary.
Exporters often face stricter requirements than domestic sellers, as many overseas markets require an Official Assurance or Free Sale Certificate, which necessitates proof of GMP compliance.

Labeling and Advertising Regulations
The most frequent compliance breach in the natural health sector relates to labeling and advertising. Under the Medicines Act 1981, specifically Sections 20, 57, and 58, there are strict prohibitions against making therapeutic claims for products that are not registered medicines.
The “Therapeutic Purpose” Trap
A therapeutic purpose includes treating or preventing disease, diagnosing disease, or altering the shape, structure, size, or weight of the human body. If a natural health product label states “Cures Arthritis” or “Treats Insomnia,” it is instantly classified as a medicine by virtue of that claim. Since the product likely does not have ministerial consent as a medicine, it becomes an unapproved medicine, and its sale is illegal.
Permissible Claims
Suppliers of Medsafe natural health products must use language that refers to supporting normal health and structure/function claims. For example:
- Prohibited: “Relieves joint pain caused by arthritis.”
- Permitted: “Supports joint mobility and comfort.”
- Prohibited: “Cures the flu.”
- Permitted: “Supports the immune system.”
Advertising encompasses all media, including websites, social media posts, and testimonials. Even if a customer writes a review claiming a product cured their ailment, the company cannot use that review in their marketing without breaching the Medicines Act.
Ensuring Consumer Safety and Product Efficacy
Beyond the legal paperwork, the core objective of regulations regarding Medsafe natural health products is consumer safety. The market has seen instances of adulteration where “natural” supplements were spiked with prescription drugs (such as erectile dysfunction drugs or steroids) to make them effective. Medsafe actively monitors for such breaches.
Contaminant Testing
Responsible manufacturers must test for contaminants. This is particularly relevant for herbal medicines which can be susceptible to environmental pollution.
- Heavy Metals: Lead, mercury, cadmium, and arsenic testing is essential, especially for root herbs.
- Microbial Limits: Ensuring products are free from Salmonella, E. coli, and excessive mold or yeast.
- Pesticides: Verifying that raw materials meet residue limits.
Adverse Reaction Monitoring
New Zealand utilizes the Centre for Adverse Reactions Monitoring (CARM). If a consumer experiences a negative side effect from a natural health product, it should be reported to CARM. This data helps Medsafe identify patterns and issue safety alerts. You can learn more about this monitoring system via the principles of Pharmacovigilance.

Rongoā Māori and Traditional Medicine Context
For the NZ Integrated Herbal Medicine & Rongoā Māori Hub, it is vital to acknowledge the intersection of statutory law and Tikanga Māori. Rongoā Māori (traditional Māori healing) is protected under the Treaty of Waitangi (Te Tiriti o Waitangi), which guarantees Māori authority over their taonga (treasures), including traditional knowledge and healing practices.
Historically, Rongoā practitioners preparing remedies for individual patients have operated within a different context than mass-market commercial manufacturers. However, when Rongoā products are commercialized, packaged, and sold to the general public on retail shelves or online, they generally fall under the ambit of the Dietary Supplements Regulations or the Medicines Act, depending on the claims made.
There is ongoing dialogue regarding how to best regulate natural health products in a way that respects traditional knowledge while ensuring safety. The repeal of the Therapeutic Products Bill in early 2024 has returned the focus to the existing 1981/1985 legislation, but the sector anticipates future reforms that will better accommodate the nuances of traditional medicine while maintaining high safety standards.
People Also Ask
Does Medsafe approve natural health products?
No, Medsafe does not pre-approve natural health products or dietary supplements before they go on sale. However, they monitor the market to ensure products are safe and do not make illegal therapeutic claims.
Can I claim my herbal product cures diseases?
No. Under the Medicines Act 1981, you cannot claim a natural health product cures, treats, or prevents disease unless it has been registered as a medicine with Ministerial consent.
What is the difference between a dietary supplement and a medicine in NZ?
A medicine is intended to treat or prevent disease and requires approval. A dietary supplement is intended to supplement the diet and support general health, and it does not require pre-market approval but must comply with specific regulations.
Are Rongoā Māori products regulated by Medsafe?
When Rongoā Māori products are commercialized and sold to the public, they are subject to the same regulations as other natural health products regarding safety and claims. Traditional practice between a healer and patient often operates under different cultural protocols.
How do I report an adverse reaction to a natural health product?
Adverse reactions should be reported to the Centre for Adverse Reactions Monitoring (CARM) in New Zealand, which works with Medsafe to monitor product safety.
What regulations apply to importing natural health products into NZ?
Imported products for personal use are generally permitted if they are not prescription medicines. However, importing for commercial sale requires strict adherence to the Dietary Supplements Regulations and the Medicines Act.
