To report herbal side effects NZ, you must utilize the Centre for Adverse Reactions Monitoring (CARM) database, which is overseen by Medsafe. Both consumers and healthcare professionals can submit reports regarding adverse events from natural health products, dietary supplements, and Rongoā Māori via the online reporting tool on the CARM website or by completing a physical “Yellow Card” form. Prompt reporting is essential for identifying safety signals and protecting public health.
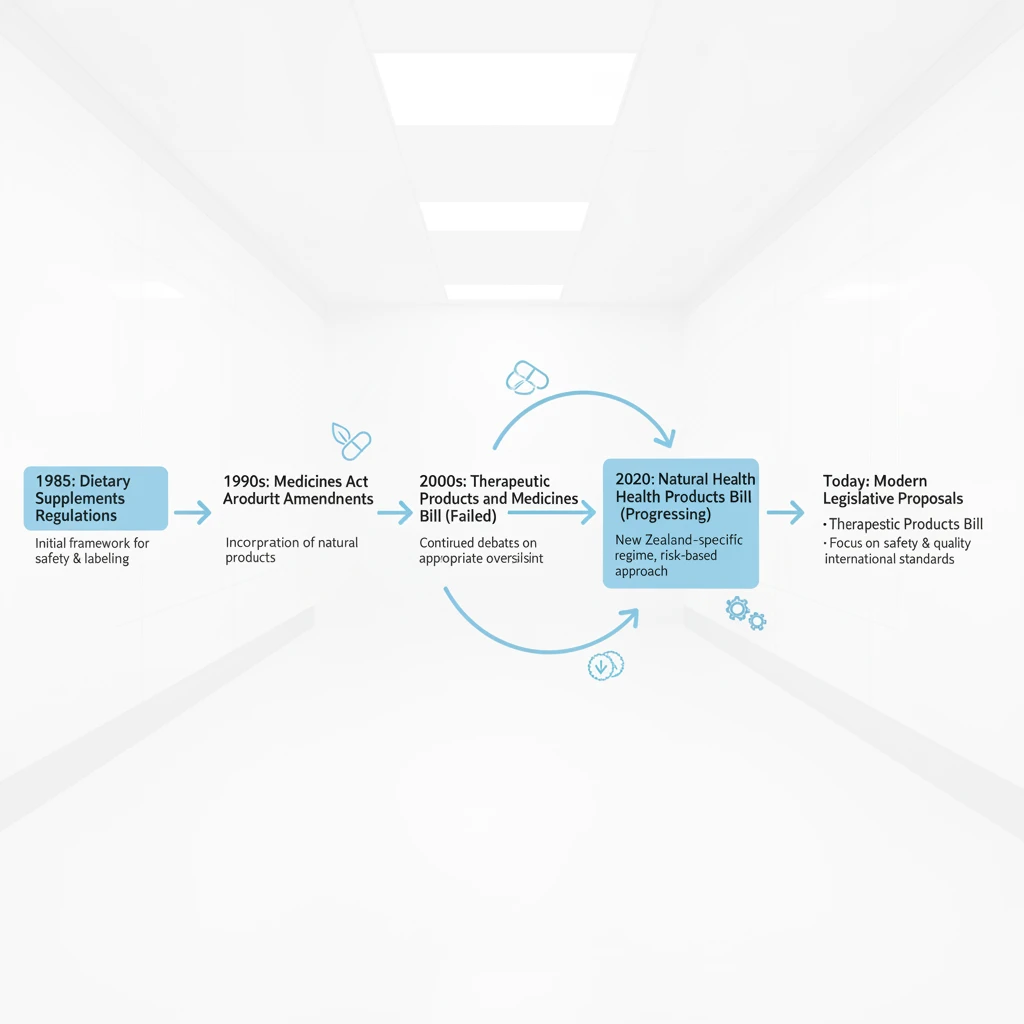
New Zealand has a rich culture of utilizing natural medicine, ranging from traditional Rongoā Māori practices to modern Western herbal medicine and dietary supplements. While many New Zealanders rely on these products for maintaining wellness, there is a pervasive misconception that “natural” always equates to “safe.” Like pharmaceutical drugs, herbal products contain pharmacologically active compounds that can cause side effects, toxicity, or serious interactions with prescription medications.
Ensuring the safety of the herbal medicine sector relies heavily on pharmacovigilance—the practice of monitoring the effects of medical products after they have been licensed for use. In New Zealand, the mechanism for this is robust, yet underutilized by the general public. Understanding how to recognize an adverse event and knowing how to report herbal side effects NZ authorities require is a critical skill for any consumer or practitioner of natural health visiting our Home page.
Understanding Adverse Reactions to Herbal Medicines
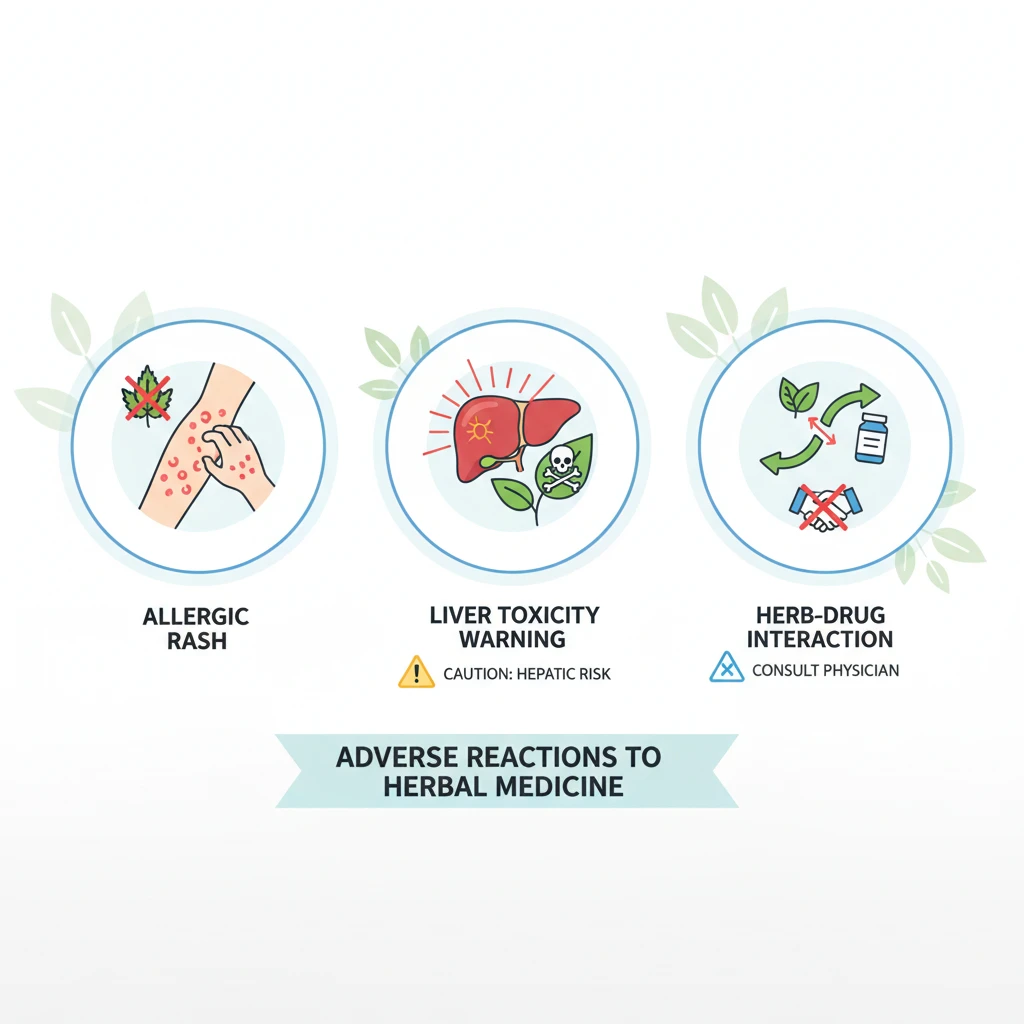
An adverse reaction is any unintended or harmful response to a health product. With herbal medicines, these reactions can be complex due to the variability in plant material, extraction methods (as seen in Herbal Vinegars: Uses and Preparation with Native Botanicals), and formulation quality. Unlike synthetic drugs, which usually contain a single active ingredient, herbs contain hundreds of phytochemicals that may affect the body in different ways.
Adverse reactions generally fall into several categories:
- Allergic Reactions: These can range from mild skin rashes (urticaria) to severe anaphylaxis. For example, individuals sensitive to the Asteraceae family (daisies) may react to Echinacea or Chamomile.
- Toxic Reactions: These occur when a herb causes direct damage to an organ, most commonly the liver (hepatotoxicity) or kidneys (nephrotoxicity). This can happen due to incorrect dosage, prolonged use, or contamination with heavy metals or wrong plant species.
- Idiosyncratic Reactions: Unpredictable reactions that occur in a small number of people due to genetic differences in how they metabolize the herb, which can be linked to your unique constitution, such as when Decoding Your Dosha: Vata, Pitta, Kapha.
- Herb-Drug Interactions: This is the most common safety concern. A herb may speed up or slow down the metabolism of a prescription drug, leading to treatment failure or overdose of the pharmaceutical.
The Medsafe and CARM Reporting System Explained
In New Zealand, the regulation and monitoring of medicines fall under the jurisdiction of Medsafe (New Zealand Medicines and Medical Devices Safety Authority), a business unit of the Ministry of Health. However, the actual collection and analysis of adverse reaction reports are handled by the Centre for Adverse Reactions Monitoring (CARM), based at the University of Otago in Dunedin.
CARM maintains the national database of adverse reactions. This database is a vital tool for identifying safety signals. A “safety signal” is information on a new or known adverse event that is potentially caused by a medicine and that warrants further investigation. Because clinical trials for herbal products are rarely as extensive as those for pharmaceuticals, post-market monitoring (reporting by users) is often the only way rare or serious side effects are discovered.
It is important to note that you do not need to be certain that the herbal product caused the reaction. A suspicion is sufficient grounds to file a report. CARM’s medical assessors will evaluate the data to determine the likelihood of a causal link.
How to Submit a Report: A Step-by-Step Guide
Reporting a side effect is a straightforward process designed to be accessible to both healthcare professionals and the general public. Here is how you can submit a report regarding a herbal product or supplement.
1. Gather Necessary Information
Before starting the report, collect as much detail as possible. This increases the value of the data for researchers. Try to have the following on hand:
- Product Details: Brand name, ingredients list, batch number, and expiry date.
- Dosage: How much was taken, how often, and for how long.
- The Reaction: Description of symptoms, when they started, and how long they lasted.
- Patient Details: Age, gender, and any other medications being taken (to check for interactions).
2. Choose Your Reporting Method
There are two primary ways to submit your report to CARM:
- Online Reporting: Navigate to the CARM Reporting website. There are specific portals for “Consumers” and “Healthcare Professionals.” The digital form guides you through the required fields.
- Yellow Card (Physical Form): You can download a PDF of the reporting form (often referred to as the Yellow Card) from the Medsafe website, print it, fill it out, and mail it to the address provided in Dunedin.
3. Submission and Follow-up
Once submitted, your report is entered into the database. You may be contacted by CARM if they require clarification. Importantly, reporting is confidential. Patient privacy is protected, and the data is used strictly for public health monitoring.

Why Reporting is Vital for New Zealand Public Health
Under-reporting is a significant issue globally, and New Zealand is no exception. It is estimated that only a small fraction of adverse drug reactions are ever reported to national centers. For herbal medicines, this rate is likely even lower due to the public perception that these products are benign.
Reporting is essential for several reasons:
- Regulatory Action: If Medsafe receives multiple reports regarding a specific product, they can take action. This might include issuing safety alerts, requiring warning labels, or, in extreme cases, removing the product from the market.
- Quality Control: Adverse reactions can sometimes be caused by contamination (e.g., adulteration with prescription drugs or heavy metals) rather than the herb itself. Reporting helps authorities identify and recall bad batches.
- Global Knowledge: Data from New Zealand feeds into the World Health Organization’s global database, contributing to worldwide safety knowledge about natural health products.
Preventing Interactions and Contraindications
Prevention is always superior to cure. Many adverse reactions reported to CARM are the result of interactions between herbal products and prescription medicines. The liver utilizes enzymes (specifically the Cytochrome P450 family) to metabolize drugs. Many herbs affect these enzymes, altering drug levels in the blood.
Common high-risk interactions include:
- St John’s Wort (Hypericum perforatum): Induces liver enzymes, significantly reducing the effectiveness of the contraceptive pill, blood thinners (warfarin), and antiretrovirals.
- Ginkgo Biloba: Can increase bleeding risk when taken with aspirin or anticoagulants.
- Kava (Piper methysticum): While culturally significant and generally safe when prepared traditionally, concentrated extracts have been linked to liver issues, especially when combined with alcohol or other hepatotoxic drugs.
Always consult your GP or a registered medical herbalist before combining treatments. For more detailed information on specific interactions, you can refer to the Medsafe Consumer Medicine Information sheets.
Rongoā Māori and Specific Safety Considerations
Rongoā Māori (traditional Māori healing) is an integral part of New Zealand’s healthcare landscape. It involves a holistic approach including wairua (spiritual), mirimiri (massage), and rākau rongoā (herbal medicine). While traditional knowledge has safeguarded the use of native plants for centuries, safety issues can arise, particularly with identification and preparation.
For instance, the native plant Tutu is highly toxic, and contamination of other plants with Tutu leaves has historically caused poisoning. Similarly, Kawakawa is widely used and generally safe, but excessive consumption can cause minor gastrointestinal upset in some individuals.
When using Rongoā, it is crucial to source rākau from experienced practitioners who can correctly identify plants and prepare them according to tikanga (customary protocols) which often include safety mechanisms not always understood by casual foragers. If an adverse reaction occurs after using traditional remedies, it should be reported to CARM just like any other medicine, ensuring that the specific preparation and source are noted.

The Role of Practitioners in Pharmacovigilance
Naturopaths, Medical Herbalists, and Rongoā practitioners play a frontline role in safety. Unlike GPs, who may not be aware a patient is taking supplements, natural health practitioners are often the first to hear about side effects. It is a professional ethical obligation for these practitioners to report adverse events to CARM.
Practitioners should encourage an open dialogue where patients feel safe disclosing all supplements they are taking. This transparency prevents “polypharmacy” issues where multiple treatments conflict with one another. By actively participating in the reporting system, the natural health community in New Zealand helps legitimize the industry and demonstrates a commitment to patient safety standards comparable to mainstream medicine.
People Also Ask
Can I report herbal side effects if I am not a doctor?
Yes, absolutely. The Centre for Adverse Reactions Monitoring (CARM) accepts and encourages reports from consumers, patients, and caregivers. You do not need a medical degree to submit a report; you only need a suspicion that a product caused a reaction.
What are the most common side effects of herbal medicines?
Common side effects include gastrointestinal issues (nausea, diarrhea, stomach pain), allergic skin reactions (rashes, hives), and headaches. More serious but rarer side effects can include heart palpitations, elevated blood pressure, and liver toxicity.
How do I know if my herbal supplement is interacting with my medication?
Signs of interaction can include the return of symptoms that your medication was treating (indicating the herb made the drug less effective) or increased side effects from your medication (indicating the herb increased the drug’s potency). Always check with a pharmacist or doctor.
Is it safe to take Rongoā Māori with prescription drugs?
It depends on the specific plant and the drug. While many Rongoā treatments are compatible with pharmaceuticals, some native plants can interact with medicines. It is vital to consult with a knowledgeable Rongoā practitioner and your GP to coordinate care safely.
Does Medsafe regulate all herbal products in NZ?
Currently, many herbal products fall under dietary supplement regulations rather than the Medicines Act, meaning they do not undergo the same pre-market testing as drugs. This makes post-market reporting to CARM even more important for monitoring safety.
What happens after I submit a report to CARM?
Your report is entered into the national database and analyzed by medical assessors to determine if there is a causal link. If a pattern of similar reports emerges, Medsafe may investigate further, issue safety alerts, or recall the product to protect the public.